
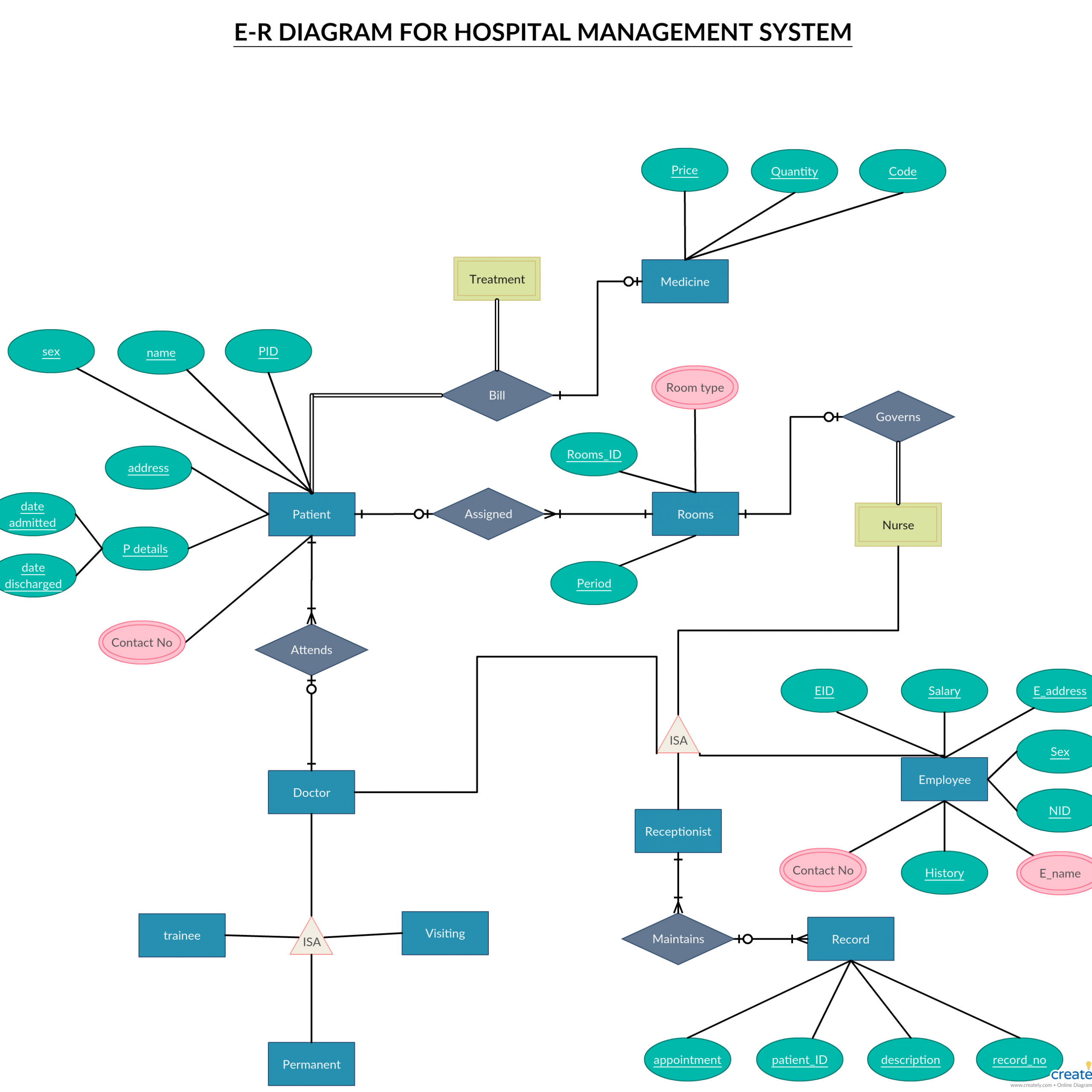
If you can understand the foundation and skeleton of the diagram specific to that molecule, then it will be easier and faster for you to draw it.Įnergy is on the y axis. The key is to first identify what molecule they are asking you to draw and then determine which of the following categories it belongs to.
Rather, we are considering the weighted linear sum of all atoms in a molecule. Put simply, the valence electrons are not confined to individual bonds.
On the other hand, Molecular Orbital Theory visions the electrons of a covalent bond to be delocalized over the entire molecule. Valence Bond Theory proposes that electrons are localized between two atoms. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory.
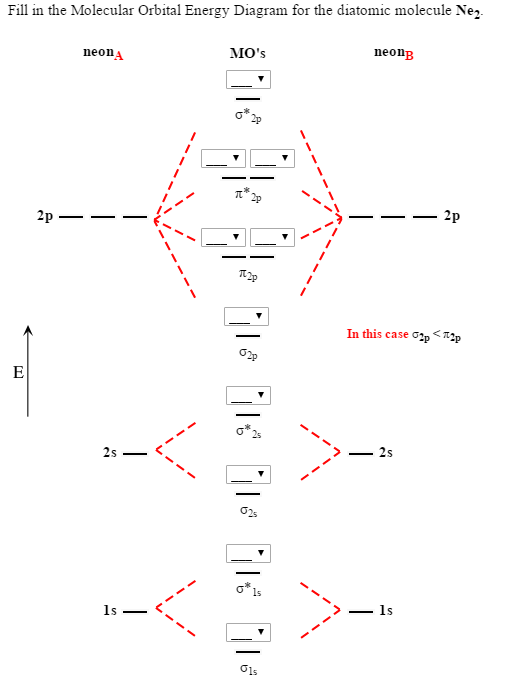
Drawing molecular orbital diagrams is one of the trickier concepts in chemistry.


 0 kommentar(er)
0 kommentar(er)
